Selected Publications
Impact of supercharging on top-down characterization of monoclonal antibodies by collision induced dissociation
Amunugama, L.; Shaw, J. B. Int. J. Mass Spectrom. 2026, 521, 117578.
In this study, we showed how supercharging from native electrospray conditions improves detection of intact mAbs and produces abundant light chain decongested MS/MS spectra using CID.

Native Top–Down Analysis of Membrane Protein Complexes Directly From In Vitro and Native Membranes
Jung et al. Mol. Cell. Proteomics 2025, 24, 100993
An exciting collaborative project with Kallol Gupta's lab at Yale School of Medicine in which we identify and characterized membrane proteins directly from membrane vesicles.

Protein Complex Heterogeneity and Topology Revealed by Electron Capture Charge Reduction and Surface Induced Dissociation
Shaw, J. B. [Co-Corresponding Author] et al. ACS Cent. Sci. 2024, 10, 1537–1547
Here, we demonstrate the utility of native MS combined with gas phase charge reduction and surface induced dissociation for the characterization of protein complex topology and heterogeneity

Enhanced Top-Down Protein Characterization with Electron Capture Dissociation and Cyclic Ion Mobility Spectrometry
Shaw, J. B. [Corresponding Author] et al. Anal. Chem. 2022, 94, 3888–3896.
In this study, we use ion mobility spectrometry to separate ECD product ions and decongest top-down MS/MS spectra for greatly increased protein sequence coverage and other figures of merit.

Charge Movement and Structural Changes in the Gas-Phase Unfolding of Multimeric Protein Complexes Captured by Native Top-Down Mass Spectrometry
Zhou, M; Liu, W; Shaw, J. B. [Corresponding Author] Anal. Chem. 2020, 92, 1788-1795.
In this study, we use electron capture dissociation and ultraviolet photodissociation to track gas-phase unfolding of protein complexes with single amino acid resolution.

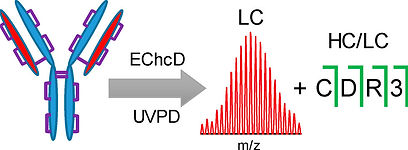
Direct Determination of Antibody Chain Pairing by Top-down and Middle-down Mass Spectrometry Using Electron Capture Dissociation and Ultraviolet Photodissociation
Shaw, J. B. [Corresponding Author] et al. Anal. Chem. 2020, 92, 766-773.
In this study, we developed methodology for determining antibody chain pairing from a single top-down or middle-down mass spectrum.
Sequencing Grade Tandem Mass Spectrometry for Top–Down Proteomics Using Hybrid Electron Capture Dissociation Methods in a Benchtop Orbitrap Mass Spectrometer
Shaw, J. B. [Corresponding Author] et al. Anal. Chem. 2018, 90, 10819-10827.
In this work, we developed and implemented a new ExD device for fast and more extensive characterization of intact proteins. These gains extended to antibody sequence characterization.

Complete Protein Characterization Using Top-Down Mass Spectrometry and Ultraviolet Photodissociation
Shaw, J. B. et al. J. Am. Chem. Soc. 2013, 135, 12646-12651.
In this work, we implemented 193 nm ultraviolet photodissociation (UVPD) in an Orbitrap mass spectrometer for top-down protein characterization. We demonstrated unprecedent capabilities for intact protein characterization.

Complete List of Publications
Most recent listed first
-
Amunugama, L.; Shaw, J. B. Impact of Supercharging on Top-down Characterization of Monoclonal Antibodies by Collision Induced Dissociation. International Journal of Mass Spectrometry 2026, 521, 117578. https://doi.org/10.1016/j.ijms.2026.117578.
-
Jung, W.; Panda, A.; Lee, J.; Ghosh, S.; Shaw, J. B.; Gupta, K. Native Top-down Analysis of Membrane Protein Complexes Directly from in Vitro and Native Membranes. Molecular & Cellular Proteomics 2025, 24 (7), 100993. https://doi.org/10.1016/j.mcpro.2025.100993.
-
Shaw, J. B. [Co-Corresponding Author]; Harvey, S. R.; Du, C.; Xu, Z.; Edgington, R. M.; Olmedillas, E.; Saphire, E. O.; Wysocki, V. H. Protein Complex Heterogeneity and Topology Revealed by Electron Capture Charge Reduction and Surface Induced Dissociation. ACS Cent. Sci. 2024, 10 (8), 1537–1547. https://doi.org/10.1021/acscentsci.4c00461.
-
Lantz, C.; Schrader, R.; Meeuwsen, J.; Shaw, J.; Goldberg, N. T.; Tichy, S.; Beckman, J.; Russell, D. H. Digital Quadrupole Isolation and Electron Capture Dissociation on an Extended Mass Range Q-TOF Provides Sequence and Structure Information on Proteins and Protein Complexes. J. Am. Soc. Mass Spectrom. 2023, 34 (8), 1753–1760. https://doi.org/10.1021/jasms.3c00184.
-
Shaw, J. B. [Corresponding Author]; Cooper-Shepherd, D. A.; Hewitt, D.; Wildgoose, J. L.; Beckman, J. S.; Langridge, J. I.; Voinov, V. G. Enhanced Top-Down Protein Characterization with Electron Capture Dissociation and Cyclic Ion Mobility Spectrometry. Anal. Chem. 2022, 94 (9), 3888–3896. https://doi.org/10.1021/acs.analchem.1c04870.
-
Zhou, M.; Lee, J. Y.; Park, G. W.; Malhan, N.; Liu, T.; Shaw, J. B. [Corresponding Author] Evaluating the Performance of 193 Nm Ultraviolet Photodissociation for Tandem Mass Tag Labeled Peptides. Analytica 2021, 2 (4), 140–155. https://doi.org/10.3390/analytica2040014.
-
Shaw, J. B. Direct Determination of Antibody Chain Pairing. US Patent 11,152,198, 2021, October 19, 2021.
-
Novikova, I. V.; Zhou, M.; Du, C.; Parra, M.; Kim, D. N.; VanAernum, Z. L.; Shaw, J. B.; Hellmann, H.; Wysocki, V. H.; Evans, J. E. Tunable Heteroassembly of a Plant Pseudoenzyme–Enzyme Complex. ACS Chem. Biol. 2021, 16 (11), 2315–2325. https://doi.org/10.1021/acschembio.1c00475.
-
Gilchuk, P. et al. Proteo-Genomic Analysis Identifies Two Major Sites of Vulnerability on Ebolavirus Glycoprotein for Neutralizing Antibodies in Convalescent Human Plasma. Front. Immunol. 2021, 12, 706757. https://doi.org/10.3389/fimmu.2021.706757.
-
Beckman, J. S.; Voinov, V. G.; Hare, M.; Sturgeon, D.; Vasil’ev, Y.; Oppenheimer, D.; Shaw, J. B.; Wu, S.; Glaskin, R.; Klein, C.; Schwarzer, C.; Stafford, G. Improved Protein and PTM Characterization with a Practical Electron-Based Fragmentation on Q-TOF Instruments. J. Am. Soc. Mass Spectrom. 2021, 32 (8), 2081–2091. https://doi.org/10.1021/jasms.0c00482.
-
Zhou, M.; Liu, W.; Shaw, J. B. [Corresponding Author] Charge Movement and Structural Changes in the Gas-Phase Unfolding of Multimeric Protein Complexes Captured by Native Top-Down Mass Spectrometry. Anal. Chem. 2020, 92 (2), 1788–1795. https://doi.org/10.1021/acs.analchem.9b03469.
-
Velivelli, S. L. S.; Czymmek, K. J.; Li, H.; Shaw, J. B.; Buchko, G. W.; Shah, D. M. Antifungal Symbiotic Peptide NCR044 Exhibits Unique Structure and Multifaceted Mechanisms of Action That Confer Plant Protection. Proc. Natl. Acad. Sci. U.S.A. 2020, 117 (27), 16043–16054. https://doi.org/10.1073/pnas.2003526117.
-
Srzentić, K. et al. Interlaboratory Study for Characterizing Monoclonal Antibodies by Top-Down and Middle-Down Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2020, 31 (9), 1783–1802. https://doi.org/10.1021/jasms.0c00036.
-
Shaw, J. B. [Corresponding Author]; Liu, W.; Vasil′ev, Y. V.; Bracken, C. C.; Malhan, N.; Guthals, A.; Beckman, J. S.; Voinov, V. G. Direct Determination of Antibody Chain Pairing by Top-down and Middle-down Mass Spectrometry Using Electron Capture Dissociation and Ultraviolet Photodissociation. Anal. Chem. 2020, 92 (1), 766–773. https://doi.org/10.1021/acs.analchem.9b03129.
-
Samarah, L. Z.; Khattar, R.; Tran, T. H.; Stopka, S. A.; Brantner, C. A.; Parlanti, P.; Veličković, D.; Shaw, J. B.; Agtuca, B. J.; Stacey, G.; Paša-Tolić, L.; Tolić, N.; Anderton, C. R.; Vertes, A. Single-Cell Metabolic Profiling: Metabolite Formulas from Isotopic Fine Structures in Heterogeneous Plant Cell Populations. Anal. Chem. 2020, 92 (10), 7289–7298. https://doi.org/10.1021/acs.analchem.0c00936.
-
Nagy, G.; Attah, I. K.; Conant, C. R.; Liu, W.; Garimella, S. V. B.; Gunawardena, H. P.; Shaw, J. B.; Smith, R. D.; Ibrahim, Y. M. Rapid and Simultaneous Characterization of Drug Conjugation in Heavy and Light Chains of a Monoclonal Antibody Revealed by High-Resolution Ion Mobility Separations in SLIM. Anal. Chem. 2020, 92 (7), 5004–5012. https://doi.org/10.1021/acs.analchem.9b05209.
-
Stopka, S. A.; Samarah, L. Z.; Shaw, J. B.; Liyu, A. V.; Veličković, D.; Agtuca, B. J.; Kukolj, C.; Koppenaal, D. W.; Stacey, G.; Paša-Tolić, L.; Anderton, C. R.; Vertes, A. Ambient Metabolic Profiling and Imaging of Biological Samples with Ultrahigh Molecular Resolution Using Laser Ablation Electrospray Ionization 21 Tesla FTICR Mass Spectrometry. Anal. Chem. 2019, 91 (8), 5028–5035. https://doi.org/10.1021/acs.analchem.8b05084.
-
Novikova, I.; Zhou, M.; Shaw, J.; Hellmann, H.; Evans, J. E. Dual Native MS and Cryo-EM Approach to Resolve Heteromeric Protein Assemblies and Subunit Stoichiometry. Microsc Microanal 2019, 25 (S2), 1214–1215. https://doi.org/10.1017/S1431927619006809.
-
Boiteau, R. M.; Fansler, S. J.; Farris, Y.; Shaw, J. B.; Koppenaal, D. W.; Pasa-Tolic, L.; Jansson, J. K. Siderophore Profiling of Co-Habitating Soil Bacteria by Ultra-High Resolution Mass Spectrometry. Metallomics 2019, 11 (1), 166–175. https://doi.org/10.1039/C8MT00252E.
-
Shaw, J. B. [Corresponding Author]; Malhan, N.; Vasil’ev, Y. V.; Lopez, N. I.; Makarov, A.; Beckman, J. S.; Voinov, V. G. Sequencing Grade Tandem Mass Spectrometry for Top–Down Proteomics Using Hybrid Electron Capture Dissociation Methods in a Benchtop Orbitrap Mass Spectrometer. Anal. Chem. 2018, 90 (18), 10819–10827. https://doi.org/10.1021/acs.analchem.8b01901.
-
Shaw, J. B.; Gorshkov, M. V.; Wu, Q.; Paša-Tolić, L. High Speed Intact Protein Characterization Using 4X Frequency Multiplication, Ion Trap Harmonization, and 21 Tesla FTICR-MS. Anal. Chem. 2018, 90 (9), 5557–5562. https://doi.org/10.1021/acs.analchem.7b04606.
-
LeDuc, R. D. et al. ProForma: A Standard Proteoform Notation. J. Proteome Res. 2018, 17 (3), 1321–1325. https://doi.org/10.1021/acs.jproteome.7b00851.
-
Gargano, A. F. G.; Shaw, J. B.; Zhou, M.; Wilkins, C. S.; Fillmore, T. L.; Moore, R. J.; Somsen, G. W.; Paša-Tolić, L. Increasing the Separation Capacity of Intact Histone Proteoforms Chromatography Coupling Online Weak Cation Exchange-HILIC to Reversed Phase LC UVPD-HRMS. J. Proteome Res. 2018, 17 (11), 3731–3800. https://doi.org/10.1021/acs.jproteome.8b00458.
-
Callister, S. J.; Fillmore, T. L.; Nicora, C. D.; Shaw, J. B.; Purvine, S. O.; Orton, D. J.; White, R. A.; Moore, R. J.; Burnet, M. C.; Nakayasu, E. S.; Payne, S. H.; Jansson, J. K.; Paša-Tolić, L. Addressing the Challenge of Soil Metaproteome Complexity by Improving Metaproteome Depth of Coverage through Two-Dimensional Liquid Chromatography. Soil Biol. Biochem. 2018, 125, 290–299. https://doi.org/10.1016/j.soilbio.2018.07.018.
-
Boiteau, R. M.; Shaw, J. B.; Pasa-Tolic, L.; Koppenaal, D. W.; Jansson, J. K. Micronutrient Metal Speciation Is Controlled by Competitive Organic Chelation in Grassland Soils. Soil Biol. Biochem. 2018, 120, 283–291. https://doi.org/10.1016/j.soilbio.2018.02.018.
-
Walker, L. R.; Tfaily, M. M.; Shaw, J. B.; Hess, N. J.; Paša-Tolić, L.; Koppenaal, D. W. Unambiguous Identification and Discovery of Bacterial Siderophores by Direct Injection 21 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Metallomics 2017, 9 (1), 82–92. https://doi.org/10.1039/c6mt00201c.
-
Staley, C. et al. Diurnal Cycling of Rhizosphere Bacterial Communities Is Associated with Shifts in Carbon Metabolism. Microbiome 2017, 5 (1). https://doi.org/10.1186/s40168-017-0287-1.
-
Qiu, J.; Yu, K.; Fei, X.; Liu, Y.; Nakayasu, E. S.; Piehowski, P. D.; Shaw, J. B.; Puvar, K.; Das, C.; Liu, X.; Luo, Z.-Q. A Unique Deubiquitinase That Deconjugates Phosphoribosyl-Linked Protein Ubiquitination. Cell Res. 2017, 27, 865–881. https://doi.org/10.1038/cr.2017.66.
-
Park, J. et al. Informed-Proteomics: Open-Source Software Package for Top-down Proteomics. Nat. Methods 2017, 14 (9), 909–914. https://doi.org/10.1038/nmeth.4388.
-
Blair, S. L.; MacMillan, A. C.; Drozd, G. T.; Goldstein, A. H.; Chu, R. K.; Paša-Tolić, L.; Shaw, J. B.; Tolić, N.; Lin, P.; Laskin, J.; Laskin, A.; Nizkorodov, S. A. Molecular Characterization of Organosulfur Compounds in Biodiesel and Diesel Fuel Secondary Organic Aerosol. Environ. Sci. Technol. 2017, 51 (1), 119–127. https://doi.org/10.1021/acs.est.6b03304.
-
Shaw, J. B.; Robinson, E. W.; Paša-Tolić, L. Vacuum Ultraviolet Photodissociation and FT-ICR Mass Spectrometry: Revisited. Anal. Chem. 2016, 88 (6), 3019–3023. https://doi.org/10.1021/acs.analchem.6b00148.
-
Shaw, J. B.; Lin, T.-Y.; Leach, F. E.; Tolmachev, A. V.; Tolić, N.; Robinson, E. W.; Koppenaal, D. W.; Paša-Tolić, L. 21 Tesla Fourier Transform Ion Cyclotron Resonance Mass Spectrometer Greatly Expands Mass Spectrometry Toolbox. J. Am. Soc. Mass Spectrom. 2016, 27, 1929–1937. https://doi.org/10.1007/s13361-016-1507-9.
-
Cotham, V. C.; Shaw, J. B.; Brodbelt, J. S. High-Throughput Bioconjugation for Enhanced 193 Nm Photodissociation via Droplet Phase Initiated Ion/Ion Chemistry Using a Front-End Dual Spray Reactor. Anal. Chem. 2015, 87 (18), 9396–9402. https://doi.org/10.1021/acs.analchem.5b02242.
-
Dang, X. et al. The First Pilot Project of the Consortium for Top-down Proteomics: A Status Report. PROTEOMICS 2014, 14 (10), 1130–1140. https://doi.org/10.1002/pmic.201300438.
-
Cannon, J. R.; Cammarata, M. B.; Robotham, S. A.; Cotham, V. C.; Shaw, J. B.; Fellers, R. T.; Early, B. P.; Thomas, P. M.; Kelleher, N. L.; Brodbelt, J. S. Ultraviolet Photodissociation for Characterization of Whole Proteins on a Chromatographic Time Scale. Anal. Chem. 2014, 86 (4), 2185–2192. https://doi.org/10.1021/ac403859a.
-
Xu, Z.; Shaw, J. B.; Brodbelt, J. S. Comparison of MS/MS Methods for Characterization of DNA/Cisplatin Adducts. J. Am. Soc. Mass Spectrom. 2013, 24 (2), 265–273.
-
Shaw, J. B.; Li, W.; Holden, D. D.; Zhang, Y.; Griep-Raming, J.; Fellers, R. T.; Early, B. P.; Thomas, P. M.; Kelleher, N. L.; Brodbelt, J. S. Complete Protein Characterization Using Top-Down Mass Spectrometry and Ultraviolet Photodissociation. J. Am. Chem. Soc. 2013, 135 (34), 12646–12651. https://doi.org/10.1021/ja4029654.
-
Shaw, J. B.; Kaplan, D. A.; Brodbelt, J. S. Activated Ion Negative Electron Transfer Dissociation of Multiply Charged Peptide Anions. Anal. Chem. 2013, 85 (9), 4721–4728. https://doi.org/10.1021/ac4005315.
-
Shaw, J. B.; Brodbelt, J. S. Extending the Isotopically Resolved Mass Range of Orbitrap Mass Spectrometers. Anal. Chem. 2013, 85 (17), 8313–8318. https://doi.org/10.1021/ac401634b.
-
Madsen, J. A.; Xu, H.; Robinson, M. R.; Horton, A. P.; Shaw, J. B.; Giles, D. K.; Kaoud, T. S.; Dalby, K. N.; Trent, M. S.; Brodbelt, J. S. High-Throughput Database Search and Large-Scale Negative Polarity LC-MS/MS with Ultraviolet Photodissociation for Complex Proteomic Samples. Mol. Cell. Proteomics 2013, 10.1074/mcp.O113.028258. https://doi.org/10.1074/mcp.O113.028258.
-
Madsen, J. A.; Ko, B. J.; Xu, H.; Iwashkiw, J. A.; Robotham, S. A.; Shaw, J. B.; Feldman, M. F.; Brodbelt, J. S. Concurrent Automated Sequencing of the Glycan and Peptide Portions of O-Linked Glycopeptide Anions by Ultraviolet Photodissociation Mass Spectrometry. Anal. Chem. 2013, 85 (19), 9253–9261. https://doi.org/10.1021/ac4021177.
-
Shaw, J. B.; Madsen, J. A.; Xu, H.; Brodbelt, J. S. Systematic Comparison of Ultraviolet Photodissociation and Electron Transfer Dissociation for Peptide Anion Characterization. J. Am. Soc. Mass Spectrom. 2012, 23 (10), 1707–1715.
-
Shaw, J. B.; Ledvina, A. R.; Zhang, X.; Julian, R. R.; Brodbelt, J. S. Tyrosine Deprotonation Yields Abundant and Selective Backbone Cleavage in Peptide Anions upon Negative Electron Transfer Dissociation and Ultraviolet Photodissociation. J. Am. Chem. Soc. 2012, 134 (38), 15624–15627.
-
Han, S.-W.; Lee, S.-W.; Bahar, O.; Schwessinger, B.; Robinson, M. R.; Shaw, J. B.; Madsen, J. A.; Brodbelt, J. S.; Ronald, P. C. Tyrosine Sulfation in a Gram-Negative Bacterium. Nat. Commun. 2012, 3, 1153. https://doi.org/10.1038/ncomms2157.
-
Vasicek, L. A.; Ledvina, A. R.; Shaw, J. B.; Griep-Raming, J.; Westphall, M. S.; Coon, J. J.; Brodbelt, J. S. Implementing Photodissociation in an Orbitrap Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2011, 22 (6), 1105–1108. https://doi.org/10.1007/s13361-011-0119-7.
-
Shaw, J. B.; Brodbelt, J. S. Analysis of Protein Digests by Transmission-Mode Desorption Electrospray Ionization Mass Spectrometry with Ultraviolet Photodissociation. Int. J. Mass Spectrom. 2011, 308 (2), 203–208. https://doi.org/10.1016/j.ijms.2011.08.030.







